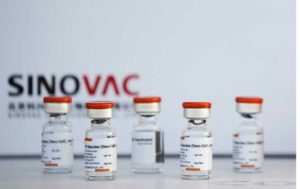
Jakarta, MINA – Indonesian Coordinating Minister for Human Development and Culture Muhadjir Effendy explained about the latest developments in the halal process for the Covid-19 vaccine made by Sinovac, which has just arrived in Indonesia.
He conveyed that the study by the Halal Product Guarantee Agency (BPJPH) and the MUI Food and Cosmetics Assessment Institute had been completed and submitted for making fatwas and MUI halal certification.
“MUI has worked hard to issue its fatwa,” said Muhadjir at the Procurement and Follow-up for the Arrival of the Covid-19 Vaccine on Monday.
He said based on Islamic jurisprudence, Covid-19 is an emergency category that must be eliminated in any way. If none of the vaccines in the world have halal status, this does not mean that the Covid-19 vaccine cannot be used.
Also Read: Peaceful Muharram: 2 Million Eid Gift Packages to Orphans and Persons with Disabilities
“So, even though the status is not halal, if it is intended to avoid emergencies, then it must be used because that emergency death must be eliminated according to religious law,” he explained.
However, if there is a vaccine that has halal status, that vaccine will be an option. As is known, the government has brought in 1.2 million doses of the Covid-19 vaccine made by Sinovac.
The vaccine is the first Covid-19 vaccine to arrive in Indonesia. However, vaccination cannot be carried out directly after the vaccine arrives, because the vaccine still has to go through several scientific stages and obtain a distribution permit from the BPOM. (T/RE1)
Mi’raj News Agency (MINA)
Also Read: Responsible Gadget Use Strengthens Healthy Digital Literacy


























![MUI Chairman for Foreign Relations and International Cooperation, Sudarnoto Abdul Hakim (center) at the One Million Women for Gaza Press Conference entitled "Women Boycott Pro-Israel Products" held at the Swiss-Belinn Cawang Hotel, East Jakarta, Thursday (3/7/2025). [Photo: Arina/MINA]](https://en.minanews.net/wp-content/uploads/2025/07/20250703_144042-scaled-1-300x225.jpg)




 Mina Indonesia
Mina Indonesia Mina Arabic
Mina Arabic